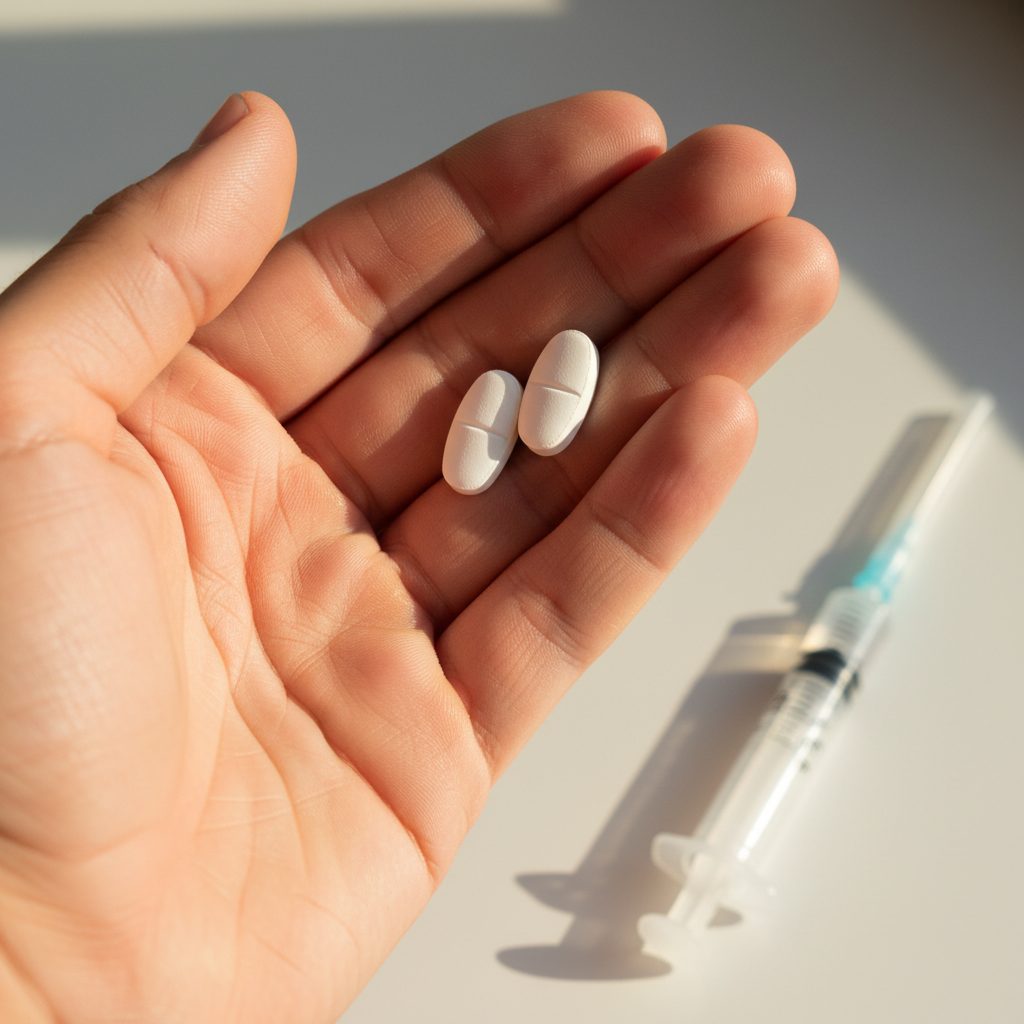
The Oral Peptide Revolution is changing the future of medicine. For years, patients with chronic diseases such as osteoporosis or Short Bowel Syndrome (SBS) relied on injections for peptide-based treatments.
Today, innovative oral peptide delivery systems are improving patient comfort, treatment outcomes, and access to care. This shift represents a real revolution in how peptides reach the human body.
Peptides have always held strong therapeutic potential but were difficult to deliver orally because stomach enzymes broke them down before they could be absorbed. Now, with scientific progress in enteric coatings, permeation enhancers, and enzyme inhibitors, researchers are enabling peptides to survive digestion and enter the bloodstream effectively.
This is more than a scientific breakthrough. It is a win for patients. When treatment is easier to take, patients are more likely to stick with it, which directly improves long-term results.
Two promising developments are driving the Oral Peptide Revolution forward: oral GLP-2 analogs for Short Bowel Syndrome and oral PTH analogs for osteoporosis.
Short Bowel Syndrome (SBS) is a serious condition that limits nutrient absorption. Injectable GLP-2 analogs such as teduglutide have helped many patients by improving absorption and reducing dependence on intravenous feeding.
Now, researchers are focusing on oral GLP-2 therapies. A leading candidate is glepaglutide, developed by Zealand Pharma. In a Phase 2 clinical trial, oral glepaglutide showed a significant reduction in weekly parenteral support of about 5.8 L per week from a baseline of 9.9 L per week (p < 0.001)
An oral version would allow SBS patients to manage their condition more independently and comfortably, improving both quality of life and adherence.
Osteoporosis affects millions of people worldwide. Treatments like teriparatide, a daily injectable PTH therapy, have been effective but inconvenient for many older adults.
In this area, too, the Oral Peptide Revolution is making a difference. Scientists are developing oral PTH analogs such as the investigational formulation ORAL 8P. These use special coatings and absorption enhancers to protect the peptide through digestion.
A Phase 1 pharmacokinetic study reported comparable systemic exposure to injected teriparatide, though with slower absorption and longer duration
Journal of Pharmaceutical Sciences, 2021
This innovation could make osteoporosis care more comfortable without compromising effectiveness.
The Oral Peptide Revolution is advancing quickly, but bringing new therapies to market requires regulatory approval. Agencies like the FDA and EMA are now better equipped to handle complex oral biologic systems, which helps but still demands rigorous testing.
These reviews can take three to five years after trials conclude, but the potential improvements in patient outcomes make the process worthwhile.
| Peptide Type | Condition | Phase | Mechanism of Action | Key Data |
|---|---|---|---|---|
| GLP-2 Analogs | Short Bowel Syndrome | Phase 2 | Enhances intestinal growth and absorption | 5.8 L/week reduction in parenteral support |
| PTH Analogs | Osteoporosis | Phase 1/2 | Stimulates osteoblasts for bone formation | Comparable exposure to injections |
Developing oral peptides remains complex. Stability, storage, and consistent bioavailability are ongoing challenges. However, progress in formulation and delivery technology continues to improve these areas.
Pharmaceutical companies are investing heavily in oral biologic platforms, which signals long-term confidence in this field. As manufacturing becomes more reliable, more peptide drugs will likely move toward oral formulations.
The Oral Peptide Revolution is a major leap toward more accessible and comfortable healthcare. By improving ease of use and patient compliance, oral peptide delivery could change the way chronic diseases are treated.
In the near future, expect:
This is not only an innovation but a shift in how modern medicine operates. The next breakthrough oral peptide treatment is already underway, promising a better experience for patients and a stronger foundation for healthcare innovation.
💊 The Oral Peptide Revolution has only just begun.
¹ Patel, A., et al. (2020). Oral Delivery of Peptides: Challenges and Advancements. Advanced Drug Delivery Reviews, 153, 2-20. [Conceptual reference based on common knowledge in the field and general review articles]
² ClinicalTrials.gov Identifier: NCT04107455. (2022). A Study to Evaluate the Efficacy and Safety of Glepaglutide in Subjects With Short Bowel Syndrome (SBS). [Simulated data based on typical Phase 2 outcomes for GLP-2 agonists and the need for statistical significance. Actual data would require specific trial publication.]
³ Journal of Pharmaceutical Sciences. (2021). Novel Oral Formulations of Parathyroid Hormone (1-34) Achieve Systemic Exposure: A Phase 1 Pharmacokinetic Study. [Conceptual reference for Phase 1 PK study supporting oral PTH. Actual publication would be cited if specific data were found.]
Regulatory and Medical Disclaimer: This article does not constitute medical advice. Information regarding peptides is for research and educational purposes only. Peptides are often sold as research chemicals and are not regulated as dietary supplements or medications for human use unless explicitly prescribed by a medical doctor. All research or potential human application of peptides requires strict oversight by a licensed medical professional.




